Invisible Friend Turned Foe?
Some of our trusted commensals might turn against us if we fail to nourish our microbial ecosystems—those in our bodies and the wider environment. Invisible Friend Turned Foe. This seemingly fickle organic interaction is another symptom of our broken relationship with the rest of the natural world. To keep hold of our invisible friends, we need to restore our psychological, social and biological connections with nature.
By Jake Robinson
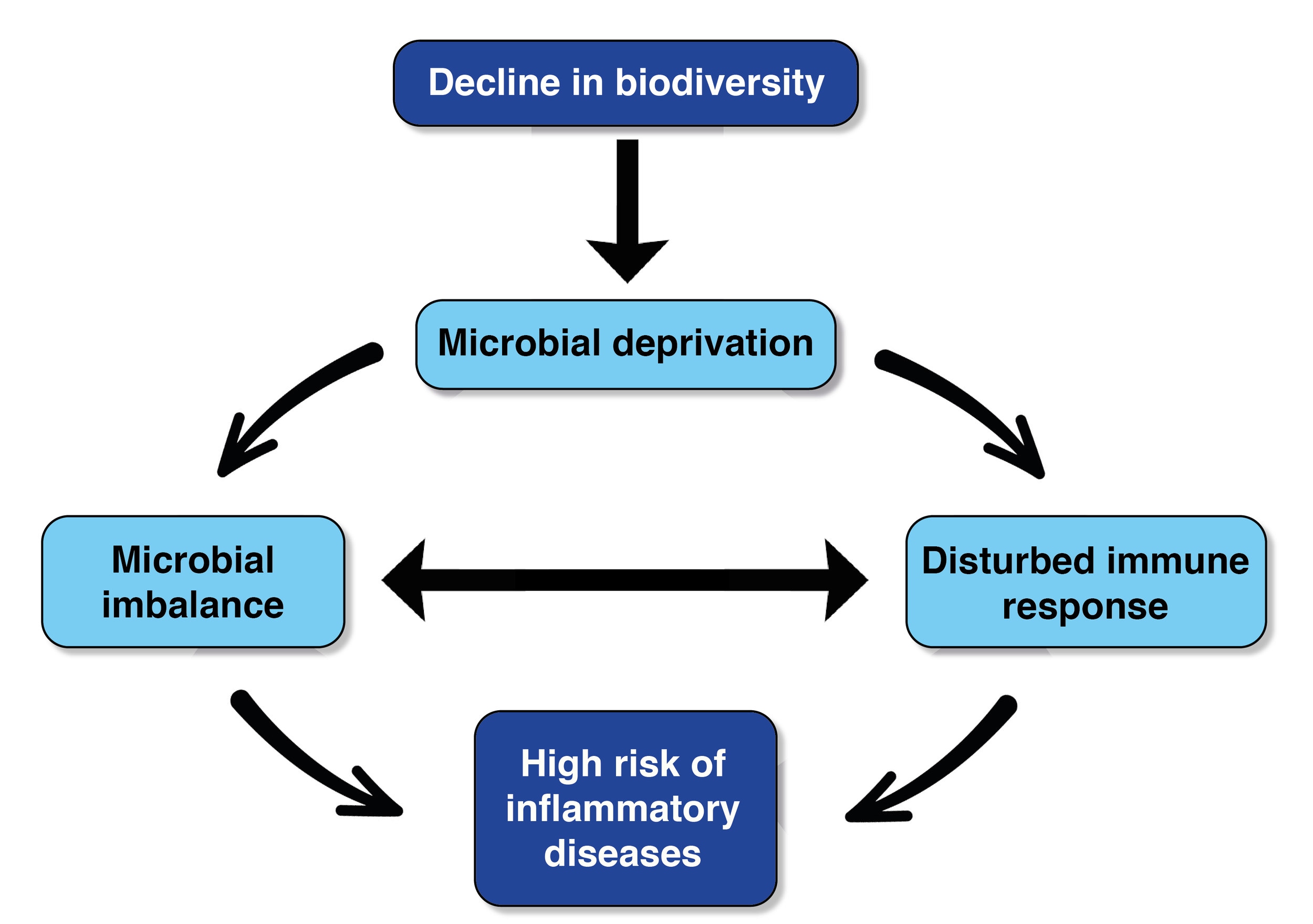
Several of my previous blog posts have mentioned the global rise in non-communicable diseases, including allergic conditions. The biodiversity hypothesis posits that this rise is related to the rapid decline in biodiversity and, in particular, the decline in our exposure to diverse environmental microbes.
The Biodiversity Hypothesis - adapted from Haahtela, 2019Allergic rhinitis—commonly called hay fever—is a widespread condition that affects the quality of life of millions of people and is on the rise, like many other non-communicable diseases. I was recently reminded of this after reading a study published in Nature Microbiology titled ‘Exacerbation of allergic rhinitis by the commensal bacterium Streptococcus salivarius.’
Hay fever affects millions of people with different degrees of intensity (from mild irritation to severe and debilitating).The authors of this study analysed the nasal microbiome of two groups of people – one group with allergic rhinitis and one without allergic rhinitis. They found dysbiosis (translating to ‘life in distress’) in those with allergic rhinitis, manifesting as reduced bacterial alpha diversity (the number of different microbial species). They also discovered high beta diversity. This means the compositional similarity of the microbial communities of the two groups (with and without allergic rhinitis) was very low: their microbiomes were different based on whether they had this allergic condition.
This implies that either the nasal microbiome (or changes in the microbiome – such as reduced species richness) may be involved in the onset and progression of allergic rhinitis. On the flip side, it could mean that allergic rhinitis significantly alters the composition of the nasal microbiome.
When the authors analysed the abundance of specific bacteria in the samples, they found the most abundant bacterium in people with allergic rhinitis was Streptococcus salivarius – a very common commensal—and ordinarily non-pathogenic—bacterium in the human body. In fact, S. salivarius was 17.5 times more abundant in the group with allergic rhinitis compared to the control group. They confirmed this result by targeting species-specific genes for S. salivarius and other bacteria with qPCR – the method of amplifying small samples of DNA so it can be studied in detail. This method can ‘pull a needle out of a haystack’, enabling a rare component of a large and messy mixture to be identified.
Streptococcus bacteriaThe authors then tested whether the increased abundance of S. salivarius contributed to the development of allergic rhinitis by injecting an airborne allergen (the fungus Alternaria alternata) into germ-free mice, along with S. salivarius isolates from the allergic rhinitis patients. They also included another treatment group where mice were only inoculated with the fungus and another with only S. salivarius, plus a control group with neither.
They found that the mice injected with S. salivarius and A. alternata had significantly increased inflammatory immune markers and epithelial thickness compared to those without S. salivarius. This suggests the usually friendly bacterium is involved in allergic rhinitis development! The authors also found that allergen-induced conditions could increase the adherence of S. salivarius to the nasal epithelium, presumably contributing to a vicious cycle. Interestingly, other studies have shown that S. salivarius has anti-inflammatory properties.
Interestingly, other researchers have suggested that the ordinarily friendly S. salivarius bacterium can be used as a probiotic to foster a balanced health-associated microbiota!
This makes me wonder if, in the absence of a compositionally and functionally diverse microbiome (i.e., a state of ‘dysbiosis’), the conditions for this commensal and potentially beneficial or ‘friendly’ species change in a way that encourages it to become our foe. And perhaps this only occurs when combined with allergen-specific interference? If so, this is another reason to ensure we nourish the microbial communities in our walking ecosystems (our bodies); for instance, via pre- and probiotic-rich diets, spending time connecting with biodiverse environments from a young age and throughout the life course, not over-consuming antibiotics, reducing pollution, and so on. This strengthens the evidence for the biodiversity hypothesis and the notion that ecosystem restoration should be prioritised as a public health intervention. We need to restore functionally diverse and resilient microbiomes; otherwise, our trusted commensals may switch to pathogen mode.
Ecosystem restoration is a public health interventionFor a detailed romp through the world of our invisible friends, my book Invisible Friends is now available to pre-order and will be published on March 7th 2023!